By Amy Young, UC Davis Center for Equine Health
The first equine genetic tests became available in the 1990s, well before the equine genome sequence was completed in 2009. Technical advances have since led to a rapid expansion in available tests. Here are a few that can be utilized to inform breeding decisions and provide diagnostic information.
Appaloosas
Some Appaloosas are closely related to Quarter Horses, so the AQHA five panel test (add hyperlink) is recommended.
Equine recurrent uveitis (ERU) is characterized by inflammation of the middle layer of one or both eyes. Cumulative damage can lead to cataracts, glaucoma, and blindness. Appaloosas are eight times more likely to develop ERU than other breeds and significantly more likely to become blind. It is thought to be a complex disorder. However, research identified the leopard complex white spotting pattern (LP) allele as an associated ERU risk factor, with homozygotes (LP/LP) – inheriting the same allele from both parents - being at highest risk. Genetic testing can be used to identify which horses should be examined more frequently. Infectious organisms, particularly Leptospira spp. have also been associated with ERU. There is no cure, and early diagnosis and intervention are associated with the best prognosis.

An Appaloosa homozygous for leopard complex white spotting (LP/LP). Photo courtesy of UC Davis
Congenital stationary night blindness (CSNB) is the inability to see in low to no-light conditions. Horses with LP/LP have abnormal cell signaling from the rods, or low light detecting cells of the retina, to the next cell in the visual pathway. Affected horses likely have normal vision during daylight, but may exhibit anxiety, apprehension, and confusion in low light. They may bump into things, be reluctant to move, or be prone to injury at night. There is no cure for CSNB, but most horses can be managed successfully. Genetic testing can be used to confirm diagnosis.
A genetic variant associated with CSNB in Tennessee Walking Horses (CSNB2) has recently been identified. The genetic test can be used to inform mating decisions and aid in diagnosis.
Related: The "Gen-Ethics" of Equine Breeding
Arabians
Severe combined immunodeficiency (SCID) causes affected foals to be born with severely weakened immune systems. They do not produce functional B and T lymphocytes (each a specific type of white blood cell), and cannot mount appropriate immune responses to challenges, making them highly susceptible to infections. There is no cure, and affected foals die or are euthanized within the first six months of life.

Photo: Canstock/Melory
Lavender foal syndrome (LFS) causes affected foals to be unable to stand or nurse properly due to neurological impairments including seizures, hyperextension of limbs, neck, and back, and involuntary eye movements. They are born with a characteristic dilute coat colour described as lavender or silver. Supportive care can provide temporary relief in some cases, but the condition is ultimately untreatable. Affected foals die or are euthanized shortly after birth. Genetic testing can be used to confirm diagnosis and identify carriers.
Cerebellar abiotrophy (CA) is caused by the progressive death of neurons in the cerebellum. Clinical signs are variable, and can include head tremor, ataxia, exaggerated movement, a wide-based stance, and inability to rise. There is no treatment for CA. Foals that show signs of CA are euthanized or restricted to life as pasture pets, as they are never coordinated enough to be ridden safely. Affected horses are also a danger to themselves because they are predisposed to accidents and injury.
Occipitoatlantoaxial malformation (OAAM) results from the malformation of the first two vertebrae of the neck, the atlas (C1) and the axis (C2), and the base of the skull (occipital bone), with the atlas fused to the occipital bone. This causes compression and damage of the upper part of the spinal cord. Clinical signs include abnormal head and neck carriage, reluctance to move the neck, or neck twisting. There is no treatment, and affected foals are usually euthanized. There is extensive variability to this disease, so the term OAAM describes not one disease, but a group of likely inherited malformations. There is currently one genetic test for OAAM1 that can assist in identifying carriers to help with mating decisions. The genetic basis for other forms of OAAM is under investigation.

A week-old OAAM affected Arabian filly (left) with an arrow indicating the asymmetric atlas, and an unaffected foal (right). Photos courtesy of UC Davis
Related: Equine Neurological Dysfunction
Junctional epidermolysis bullosa (JEB) is a progressive skin disorder in Belgian Draft Horses and American Saddlebreds. Affected foals develop blistering and skin lesions at pressure points that worsen with time, leaving them susceptible to severe infections. A mutation (JEB1) has been identified in Belgians and related breeds. A different mutation (JEB2) is responsible for the condition in American Saddlebreds. There is no treatment for JEB and most affected foals are euthanized.

Photo: Canstock/JKLM56
Polysaccharide storage myopathy 1 (PSSM1) is found in some draft horse breeds at risk of developing PSSM1. The signs, treatment, and causative mutation are the same as described for PSSM1 as part of the AQHA five panel test.
Ocular squamous cell carcinoma (SCC) is the most common form of cancer to affect the eyes and eyelids of horses. The tumour arises in the outermost layer of skin, conjunctival, or corneal cells, with UV light (sunlight) exposure being a known risk factor. Tumours may grow rapidly and spread to adjacent tissues, causing visual impairment and destruction of the eye. Treatment options and prognosis are determined by the size and location of the tumour(s). Certain breeds, including Belgians and Haflingers, have a genetic predisposition to ocular SCC development and a major genetic risk factor has been identified. The genetic test is helpful to identify which horses are at highest risk and should be examined more frequently.
Related: The Not-So-Fab-Four - Diseases Resulting in Hind Limb Gait Deficits
Friesians
Hydrocephalus is caused by the excessive accumulation of cerebrospinal fluid in the brain, resulting in severe distension of the head, giving it a large, domed appearance. Hydrocephalus is thought to occur due to an abnormal narrowing of the opening at the base of the skull. Friesian foals affected with hydrocephalus are aborted, stillborn, or born with severe neurological issues that warrant euthanasia. A causative genetic mutation has been identified, and testing can assist in identifying carriers.

A dwarf Friesian. Photo courtesy of UC Davis
Dwarfism is characterized by disproportionate growth with reduced bone length of limbs and ribs. Affected horses exhibit severely shortened stature, shortened limbs relative to body size, bowed forelegs, a shortened neck, broad chest, and reduced bodyweight. A causative genetic mutation has been identified, and testing can assist in identifying carriers. There is no treatment, but affected foals do grow albeit at a slower rate than their unaffected counterparts. Most are able to walk, trot, canter, and gallop, and some are even ridden.
Related: Distichiasis in Friesian Horses
Pony Breeds
Foal immunodeficiency syndrome (FIS or Fell Pony syndrome) is found in rare native U.K. Fell and Dales Pony breeds and crosses. It causes fatal anemia and a compromised immune system. Affected foals have abnormally low levels of red blood cells and B-lymphocytes. They become progressively anemic and lack the ability to produce their own antibodies, which makes them susceptible to infections. The genetic test is helpful to identify carriers or confirm clinical diagnosis. There is no effective treatment for FIS and affected foals usually die or are euthanized by four years of age.

A Connemara pony Stallion. Wiki/Satu Pitkänen
Connemara hoof wall separation disease (HWSD) is characterized by separation and cracking of the outer hoof wall. This can lead to ponies supporting their weight on the sole of the hoof instead of the hoof wall, which can result in chronic inflammation, severe lameness, and laminitis. There is no treatment or cure for HWSD. Management through hoof care and/or special shoes may be attempted, but these options are expensive and labour-intensive. Affected animals can experience severe pain despite careful management. Quality of life may diminish and euthanasia may be necessary. Even if the condition is initially controllable, ponies may still develop laminitis over time. The genetic test can be used to test mating pairs to avoid producing affected offspring.
Warmbloods and Thoroughbreds
Warmblood fragile foal syndrome type 1 (WFFS) is a defect of connective tissue characterized by hyperextensible, abnormally thin, fragile skin, and mucous membranes that are subject to open lesions. Affected horses may also have hyperextensible limb joints, floppy ears, accumulation of fluid (hydrops), subcutaneous emphysema, hematomas, and premature birth. Newborn foals are euthanized shortly after birth due to the poor prognosis. Due to the severity of the disease, there is no treatment or cure. Genetic testing prior to mating is advisable to avoid producing affected offspring. There are no known health problems associated with carrier status. This allele was detected in the Thoroughbred at a low frequency, so testing is also recommended for that breed.

Photo: Shutterstock/Grigorita KO
Equine familial isolated hypoparathyroidism (EFIH) is an inherited form of hypocalcemia, or low calcium concentrations in the blood, and has been identified in Thoroughbred foals. Hypocalcemia can impair limb movement and weaken bones. In severe cases, it can lead to seizures, muscle fasciculations, intestinal problems, heart issues, and ataxia. Genetic testing is now available through the UC Davis Veterinary Genetics Laboratory.
Related: 10 Facts About Equine Genetics
HOW GENETIC DISEASES ARE INHERITED
Modes of Inheritance
Most identified equine genetic diseases have autosomal dominant or recessive modes of inheritance. “Autosomal” means that males and females are equally affected. Here, “unaffected” and “affected” represent the phenotype, or presence of the disease. Aa, aa, BB, Bb, and bb represent the genotype, or genetic combination of alleles (one from each parent) that causes the phenotype.
Autosomal Dominant
ONE copy of the gene variant (allele) is needed to have the disease/trait. Each offspring of an unaffected parent x affected parent has a 50 percent chance of inheriting the disease allele (A) and being affected.
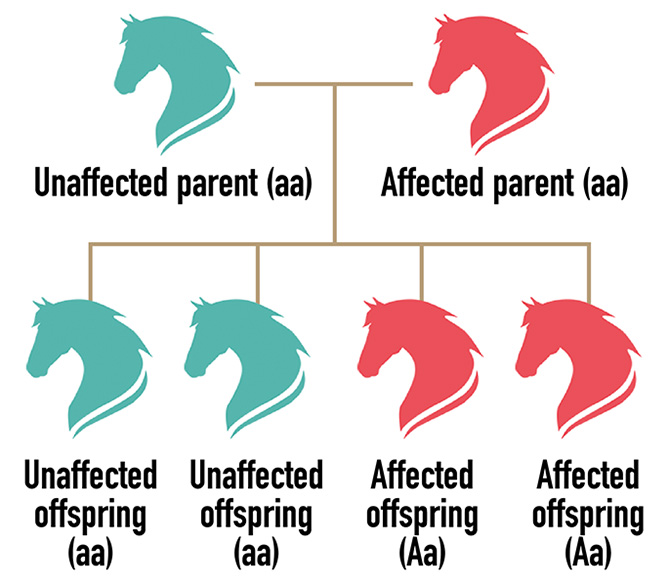
Autosomal Recessive
TWO copies of the gene variant (allele) are needed to have the disease/trait. Each offspring of parents who carry the condition has a 25 percent chance of inheriting the disease allele and being affected. Carriers are unaffected but can pass the disease/trait to their offspring.
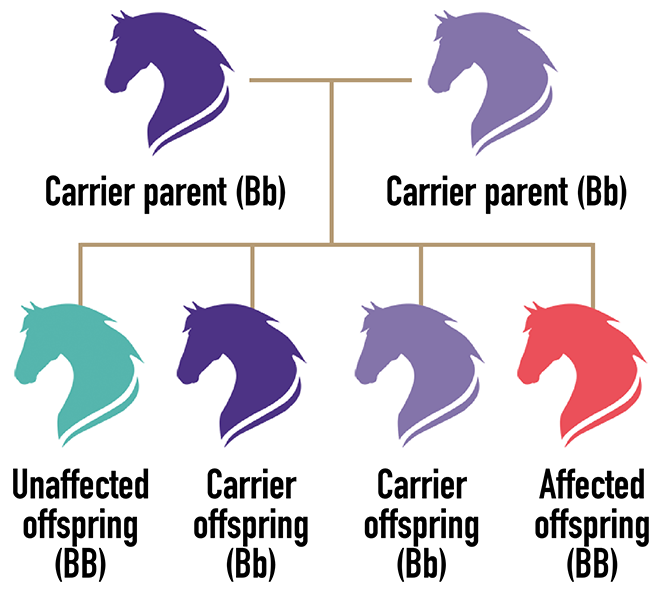
Related: AQHA's Dedication to Research and Success of the Five-Panel Test
Published with the kind permission of the UC Davis Center for Equine Health. The UC Davis Center for Equine Health is dedicated to advancing the health, welfare, performance and veterinary care of horses through research, education and public service.
Main Photo: Slowmotiongli




























